Embryonic Stem Cells Proliferate and Differentiate when Seeded into Kidney Scaffolds
Abstract
The scarcity of transplant allografts for diseased organs has prompted efforts at tissue regeneration using seeded scaffolds, an approach hampered by the enormity of cell types and complex architectures. Our goal was to decellularize intact organs in a manner that retained the matrix signal for differentiating pluripotent cells. We decellularized intact rat kidneys in a manner that preserved the intricate architecture and seeded them with pluripotent murine embryonic stem cells antegrade through the artery or retrograde through the ureter. Primitive precursor cells populated and proliferated within the glomerular, vascular, and tubular structures. Cells lost their embryonic appearance and expressed immunohistochemical markers for differentiation. Cells not in contact with the basement membrane matrix became apoptotic, thereby forming lumens. These observations suggest that the extracellular matrix can direct regeneration of the kidney, and studies using seeded scaffolds may help define differentiation pathways.
Introduction
Tissue engineering using stem cells to repair or reconstitute organ function is thought to hold great promise for regenerative medicine. A variety of remarkably different approaches have been proposed, and these range from tissue repair to growth of entirely new organs.1,2 There are many progenitor cell types that could potentially be used, and candidates include stem cells of embryonic origin as well as those harvested from adult organs. Stem cells have the advantage of self-replication and the potential to differentiate into multiple somatic cell types, allowing the maintenance of only a few cell lines for use in a variety of applications. Isolating the signals necessary to deliver organ-specific differentiation is at the crux of contemporary investigation, but this has been hampered because of the cellular heterogeneity, complexity of the tissues targeted for growth, and the limited, albeit evolving, state of knowledge about their nature. It is thought that the proper tissue microenvironment for support of stem cell differentiation and tissue formation would be characterized by a multitude of signals, including matrix proteins, soluble or immobilized growth factors, cytokines, cell-to-cell interactions, and mechanical forces.
To address these issues various scaffolds have been used as a framework for precursor cells. Those that are synthetic, however, typically do not have the intricate architecture needed for whole organs, do not provide signals for differentiation, and thus have had limited success. Scaffolds made from biological tissues would be appealing if they could be prepared to remove all native cells but still retain the extracellular matrix (ECM) necessary for signaling. Appropriate choices for the donor scaffold and precursor cells would thus be the basis of achieving the long-term goal of regenerating an organ of limited immunogenicity.3 Indeed, decellularized tissue scaffolds have been tried for direct implantation (relying on the presence of endogenous precursor cells), but they have been limited to relatively simple structures such as the ureter, esophagus, or heart valves.4–7 We believe that even intricate organs can be carefully decellularized to retain the innate matrix constituents needed for promoting cell adhesion, migration, proliferation, differentiation, and regulation of intercellular signaling.8 It has been postulated that only organ- derived intact ECMs capture the histoarchitecture that best represents the in vivo milieu and are in theory best able to facilitate tissue regeneration.9,10 Therefore, our novel approach was to establish an ECM-preserving decellularization method for a complex organ, seed the resultant scaffold ex vivo with embryonic stem (ES) cells and study its ability to induce tissuespecific differentiation. We chose the kidney because of its highly complicated microarchitecture, characterized by basement membranes of diverse protein composition that supports at least 26 different cell types in close apposition.11 Kidneys are also an appealing model because there is a wildly recognized hope that eventually they can be grown in vitro to address the needs of the growing number of patients suffering from renal failure, the lack of available organs for transplantation, and the inadequacies and expense of dialysis.12 Specifically, we removed all cells from rat kidneys using sequential gentle detergent, osmotic, and enzymatic steps; seeded the whole organ with murine pluripotent ES cells through the renal artery or the ureter; and incubated the whole kidney in growth media that was either static or perfused through a pulsatile system to simulate the mechanical forces (and potential signals) delivered by a native circulatory system. No prodifferentiation agents were added to the culture so that cell responses to just native heterogeneous ECM could be determined. We believe that this is the first demonstration of both proliferation and differentiation of primitive ES cells in such a complex organ scaffold structure. While being far from the ultimate goal of a functional organ suitable for transplantation, we believe that this approach provides a model for studying the signaling mechanisms for growth and differentiation between precursor cells and their matrix milieu.
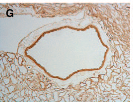
Figures: Perfusion decellularization and histology of whole rat kidneys.
- (A) Photograph of harvested kidney with arterial andureteral cannulae.
- (B) Photograph of translucent acellular kidney after SDS protocol.
- (C) Scanning electron micrograph of acellular glomerulus and adjacent tubules showing continuous basement membrane architecture (scale bar, 300 μm) after sodium deoxycholate protocol.
- (D) Renal cortex before SDS decellularization (H&E stain) .
- (E) Renal cortex after SDS decellularization showing preserved cortical ECM microstructure, with no evidence of residual nuclei or intact cells (H&E stain) .
- (F) Same as (E) with bright field.
- (G) Immunohistochemical stains verifying retention of the key basement membrane proteins: laminin in the cortical region of the scaffold.
- (H) Immunohistochemical stains verifying retention of the key basement membrane proteins: collagen IV in the medulla.






