Collaborators
Albina Mikhaylova
(Quick-Med Technologies)
Bernd Liesenfeld
(Quick-Med Technologies)
David Moore
(Quick-Med Technologies)
Jillian Vella
(Quick-Med Technologies)
Roy Carr
(Quick-Med Technologies)
Gerald Olderman
(Quick-Med Technologies)
Gregory Schultz
(Quick-Med Technologies, UF)
Summary
The dangers of bacterial colonization in wounds are well understood by caregivers – in fact the modern perspective on medical treatment can be regarded as dating back to Pasteur and the inception of aseptic techniques to prevent the infection of wounds. The challenges of resistant bacteria is that they do not respond as expected to the safety measures normally implemented to prevent bacterial infection. The challenge of resistant bacteria is complex, and an educated caregiver is more able to implement preventative and protective measures to protect themselves and those around them and in their care. Within this presentation, we explore how antimicrobial agents function, and how this relates to the mechanisms through which bacteria are able to develop resistance to those agents.
Characterization of Microbicides / Biocides
 Figure 1. Relative likelihood of developing resistance. See discussion.
Figure 1. Relative likelihood of developing resistance. See discussion.- Biocide – any substance that is specifically destructive to microbes and kills or retards the growth of microorganisms.
There are a number of types of biocides, which vary with their level of target specificity, and applicability for various situations. - Antibiotics – natural or synthetic chemical substances that have the capacity, in dilute solution, to inhibit the growth of, or to kill microorganisms.
Antibiotics are highly specialized in that they have specific cellular targets, and they require entry into a metabolically active cell in order to function. These agents represent avenues of treatment rather than methods of prevention. Because of their high degree of specificity, bacteria are most easily able to develop resistance to antibiotics. - Antiseptics – chemical agents used externally on living tissue to suppress bacterial growth.
Antiseptics are applied to living tissue/skin to reduce the possibility of infection and/or sepsis – in a preventative fashion rather than as a treatment. There are many antiseptic types that work by different mechanisms. Some of the most common antiseptics include alcohols, quaternary ammonium compounds, chlorhexidines, biguanides, iodine, hydrogen peroxide, phenol compounds, and heavy metal compounds such as silver agents (the use of mercury compounds as antiseptics is largely discontinued). The goal of antiseptic agents is generally to suppress the growth of bacterial cells while leaving intact the host mammalian cells - Disinfectants = antimicrobial used on non-living surfaces to destroy microorganisms. Disinfectants are generally utilized on inert surfaces rather than on skin and tissue, because they are agents whose activity level is extremely high – these agents are not selective and can be potentially harmful or toxic to living tissue at in-use concentrations.
Mechanisms by which bacteria develop resistance to microbicidal agents
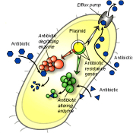
 Figure 3.Discussion.
Figure 3.Discussion.Intrinsic vs. acquired resistance. There are many instances where agents are known not to be effective against certain bacterial species – this is called natural or intrinsic resistance. Since intrinsic resistances are well documented, those agents are not used to suppress the microbes against which they are not effective. This is part of common practice by health care providers. It is acquired resistance that poses a risk in health-care and community settings because in this instance, bacterial species show decreased susceptibility to previously effective treatments. When the term resistant bacteria is used, the problem lies in the fact that it was expected the bacteria could be inhibited by some standard measure that has become ineffective.
Bacteria are able to acquire resistance to microbicidal agents by either de novo mutation, or through the acquisition of resistance genes from other bacteria. De novo mutation is also termed adaptation and is a vertical process: this represents the classically understood evolutionary process of environmental selection of individual bacteria that survive a challenge better, and thus become the progenitors of subsequent generations that carry forward this mutation that permits better survival. In contrast, the acquisition of resistance genes (these are transmitted as packets of gene sequences called plasmids) from other bacteria enable bacteria of the same generation to increase their resistance to an agent in a horizontal process [Poole, 2002].
Antiseptic agents
There are a wide variety of antiseptic agents that have been utilized as topical antimicrobials, and as active ingredients for antimicrobial wound dressings. Noted below are some small-molecule antispetic agents.
Silver agents represent the bulk of the market for the US. Silver based antimicrobial exist in various chemistries, including as metallic silver (Silvercel™ – J&J, Silverlon™ – Argentum), Silver Chloride (AquaCel Ag™ – Convatec, Silvasorb™ – Medline), and nanocrystalline silver (Acticoat™ – Smith & Nephew). Despite the diversity of silver chemistries, all silver dressings rely fundamentally on the release of silver particulates, and the accumulation of silver ions within the cells. Within the cells, silver ions poison respiratory enzymes, denature proteins and destroy cell viability by interfering with DNA replication. [Percival, 2005]
Other antiseptic agents worth noting are iodine, which is utilized both as povidone iodine, and more recently as cadexamer iodine (Iodoflex™ – Smith & Nephew.) Iodine also funtions by means of protein denaturation and nucleic acid breakdown, as well as damaging the plasma membrane – again acting on the interior of the cell.
Cationic agents
 Figure 5. Action of polymeric cationic biocidal agent Discussion.
Figure 5. Action of polymeric cationic biocidal agent Discussion. Figure 6a. E. coli on untreated gauze Discussion.
Figure 6a. E. coli on untreated gauze Discussion. Figure 6b. E. coli on NIMBUS treated gauze. Discussion.
Figure 6b. E. coli on NIMBUS treated gauze. Discussion.
Cationic antiseptics include a range of agents that can be chemically distinguished through the number of charge sites resident on the molecule (or molecular repeat unit for polymeric agents). Monocationic quaternary ammonium compounds (QACs) are best known through the common antiseptic Benzalkonium Chloride (one commercial product based on this chemistry is Bactine™ (Bayer)). Bisbiguanides have two cationic charges: Chlorhexidine is an example of this chemistry. Polyhexamethylene Biguanide (PHMB) is a small polymeric biguanide that is used in contact lens solutions and is the active antimicrobial in Kerlix AMD™ (Kendall).
Quaternary ammonium compounds (‘quats’ or polyquats in the case of polymeric structures) have a fundamentally different mechanism of antimicrobial activity from small – molecule antiseptic agents such as silver or iodine, that require entry into the cell in order to exert antimicrobial activity. Quats chemically destabilize the cell wall structures, inducing cellular collapse, as demonstrated by the SEM images in Figure 6. The chemistry of the cell wall is relatively immutable, so the generation of resistance to this mechanism is extremely unlikely.
Gilbert and Moore describe the mechanism of cell wall disruption induced by polymeric cationic biocides in excellent detail as shown in Figure 5. They specifically describe the activity of PHMB (polyhexamethylene biguanide) – which is a polymer of ~ 2,000 daltons molecular weight. The physical size of a polymer chain precludes entry into the cell for larger polymeric biocides, demonstrating that their antimicrobial efficacy is due solely to the cell wall disruption mechanism. This is critical to the discussion of bacterial resistance, because all known acquired resistance mechanisms operate against internalized agents.
Quick-Med Technologies designed NIMBUS™ antimicrobial polymeric treatments, which utilize a long chain (molecular weight >100,000 daltons) polyquaternary agent that is permanently bound to a solid substrate. Both the large size of the polymeric agent and the physical attachment to a surface preclude entry into cells, while the high charge density provided by hundreds of quaternary repeat units ensures high biocidal activity. The design of this technology purposely minimizes opportunities for bacteria to generate resistance, thus permitting safe and effective prophylactic application. A series of experiments was carried out to substantiate the assertion that bacteria do not develop resistance, as is detailed in Figure 7.
Figures
Figure 1. The relative likelihood of bacteria being able to develop resistance to an agent is related to the target specificity of the mechanism of microbicidal action. Antibiotics are highly specific in both their targeting and mechanisms, and bacteria are most easily able to develop resistance. Disinfectants are non-specific and the dangers of bacteria developing resistance to disinfectants is very low.
Figure 2. Biocide resistance mechanisms: antibiotic agents. Bacteria have shown a number of different mechanisms to increase resistance to biocides. Image from Science Quarterly, 07-08
Antibiotic agents typically target specific metabolic processes. Defense mechanisms include alterations in the metabolic pathway to circumvent antibiotic activity, or the production of enzymes that degrade or alter the antibiotic to render it ineffective.
A resistance mechanism that is also common for small molecule antiseptics is the efflux pump: a mechanism by which the bacteria pumps the biocidal agent out of the cell through the use of a transport protein. This enables the bacteria to withstand much higher concentrations of the agent (either antibiotic or antiseptic).
Figure 3. Mechanism of resistance generation to antiseptic agents. While bacteria do not develop resistance to antiseptic agents as readily as to antibiotics, many of the same defense mechanisms are applied. The image below illustrates that the mode of action of most antiseptics is to approach the cell wall, transfer into the cell interior and act on a cellular target, as illustrated by the sequence at left. Bacteria develop resistance by implementing the mechanisms illustrated on the left, including arresting the biocidal agent at the surface by presenting a barrier that is difficult to traverse, altering the cellular targets that are being acted on, and/or by using transport proteins to eject the invading agent from the cell in a efflux pump mechanism.
Figure 4. Reshedding of bacteria into a wound from a conventional dressing. Wound fluid absorbed by a non–antimicrobial dressing serves as nutrient to grow bacteria shed by the wound. The bacteria grown in the dressing can shed back into the wound to provide reinoculation. For the antimicrobial dressing, there is no bacterial growth in the dressing, and the dressing cannot act as a source of reinoculation. Additionally, the antimicrobial dressing has the capacity to serve as an effective microbial barrier to prevent entry of exogenous bacteria, and to protect others from bacteria that may be colonizing the wound.
Figure 5. Action of polymeric cationic biocidal agent. Normal bacterial membranes (panel a) are stabilized by Ca+2 ions binding anionic charged phospholipids. NIMBUS™ quat-polymer rapidly displaces Ca+2 (panel b) leading to loss of fluidity (panel c) and eventual phase separation of different lipids. Domains in the membrane then undergo a transition to more smaller micelles. Image adapted from Gilbert and Moore, 2005.
Figures 6a and 6b. SEM imaging of E. coli on untreated gauze wound dressing (top) and on NIMBUS treated wound dressing (bottom). The control and NIMBUS treated were evaluated with high resolution SEM. Two-hundred-microliter aliquots containing 105 cells/mL of the microorganisms (E. coli ATCC 15597) were pipetted directly onto the substrates and cultured for 1 h. Samples were processed for SEM by a combination of fixation and dehydration techniques designed to preserve delicate organic structures. Imaging at high magnification allows the demonstration of changes in E. coli cells induced by the antibacterial treatment. E. coli bacteria that grew in contact with control substrate has intact membranes and full rod shape. In contrast, after 1 h exposure to the NIMBUS treated surface the individual E. coli bacteria have visible changes in membrane appearance and general morphology. Some cells show indentations or even small holes on the sides with exuding intracellular content.
Figure 7. Determination of Microbicidal Activity of NIMBUS surface and Testing of Bacterial Resistance. We evaluated changes in bacterial susceptibility to NIMBUS biocide after step-by-step adaptation training of E.coli culture to active surface of the NIMBUS dressing. The sequential assessment of the minimum inhibitory concentration (MIC) was used as additional confirmation experiment.
The selection vector was created by exposing serial passages of bacteria to NIMBUS treated surface. The three or more isolated survivor’s colonies were selected and propagated into new inoculum; and exposed to the treated substrate repeating the cycle up to ten passages. By combining the several survivor colonies we assured that selection is not accidentally limited to any one organism that avoided contact with treated surface.
Previously collected baseline MIC data for E.coli was recalibrated for finer increments in biocide concentrations and compared to MIC of E.coli survivors at each passage to detect the changes in susceptibility.
The results of these experiments demonstrated that E.coli did not become resistant to NIMBUS after a prolonged and repeated exposure. Variability in experimental MIC fell within the trend of unchallenged culture. The NIMBUS treated surface had excellent efficacy against this organism even after repeated exposure. We can conclude that the NIMBUS antimicrobial surface destroys bacteria by causing irreversible damage to bacterial membranes rather than by targeting a specific intracellular target, and therefore carries a low risk of resistance development.
References
- Schultz GS, Sibbald RG, Falanga V, Ayello EA, Dowsett C, Harding K et al. Wound bed preparation: a systematic approach to wound management. Wound Repair Regen. 2003;11 Suppl 1:S1-S28.
- Wright, BJ, Lam, K, Olson, ME, Burrell, RE, Is Antimicrobial Efficacy Sufficient? A Question Concerning the Benefits of New Dressings, Wounds, 2003; 15(5):133-142
- Poole K, Mechanism of Bacterial Biocide and Antibiotic Resistance, Journal of Applied Microbiogy, 2002:92 Suppl.:55S-64S
- Percival SL, Bowler PG, Russell D, Bacterial resistance to Silver in Wound Care, J of Hosp. Infect., 2005 May, 60(1):1-7
- Silver S. Bacterial silver resistance: molecular biology and uses and misuses of silver compounds. FEMS Microbiology Reviews. 2003; 27:341-353.
- Gilbert P and Moore LE, Cationic Antiseptics: diversity of action under a common epithet, J Appl Microbiol., 2005, 99(4):703-15
