Collaborators
Olajompo Moloye
(Department of Biomedical Engineering)
Gregory Schultz
(Department of Obstetrics and Gynecology)
W.Anthony Lee
(Division of Vascular surgery and Endovascular Therapy)
Department of Biomedical Engineering
Department of Obstetrics and Gynecology
Division of Vascular Surgery and Endovascular Therapy
Introduction
Endovascular aneurysm repair (EVAR) is a minimal invasive procedure that has been shown to prevent abdominal aortic aneurysm (AAA) rupture, reduce patient morbidity and mortality compared to open surgical repair. In this technique, a metal stent with an attached fabric graft is delivered to the aneurysm site through the femoral artery. However, endoleak (around the contact points between the stent and the aorta) and migration of the stent-graft are two major problems encountered after EVAR. Stronger tissue bonding or seal between the aorta and the implanted prosthetic devices (stent graft) is proposed to prevent this problem. This can be achieved by modifying the aortic devices currently used. In this study, we present a modified vascular graft impregnated with various concentrations of sucrose and bovine serum albumin (BSA). The delivery vehicle was poly DL-(lactide-co glycolide), a well known biodegradable polymer that has been used as a drug delivery vehicle. The effect of sucrose on the release of BSA, weight loss of the coated grafts and morphology will be presented. Basic fibroblast growth factor (bFGF) , a protein known to simulate the proliferation of vascular cells (i.e. fibroblast cells, endothelial cells and smooth muscles cells), was also impregnated within the modified graft The effect of this growth factor on vascular cell proliferation is reported. The amount of sucrose impregnated affected the release of BSA. The more sucrose impregnated, the increase in water uptake and slower the release of BSA from the modified graft. An increase in cellular proliferation was observed with grafts coated with a lower sucrose concentration within 7 days. Cellular proliferation activity of the supernatant obtained after 12 hours of release kinetics study induced significant proliferation of human dermal fibroblast. Therefore the majority of the growth factor on this modified grafts might be at or near the surface of the graft rather than the interstices of the woven graft.
MATERIALS AND METHODS
![]()
- Coated vascular grafts: 2 cm x 0.5 cm samples of vascular grafts were cut from the 4” x 4” low porosity woven vascular graft (Cooley Verisoft, Boston Scientific, and Natick, MA). The uncoated weight for each graft was recorded before coating. Coating solution for the grafts was prepared by an established water-in-oil emulsion for a solvent evaporation method used for the production of biodegradable microspheres [1]. For the first step in the preparation of these microspheres, a primary emulsion (water-in-oil emulsion) was used. The coating solution consisted of the aqueous protein solution emulsified with the polymer solution. Two different concentrations of sucrose 0.6% and 1.2% was studied to observe its effect on BSA release and the bioactivity of the growth factor encapsulated.
- Release kinetics of BSA from impregnated grafts Coated grafts were placed in a 1.5 ml microcentrifuge containing 1000 μl PBS in a hybridized incubator undergoing constant rotation at 37°C. Supernatant (500 μl) from each test tube was collected 12 hours, 24 hours, 7 days, 14 days, 21 days and 28 days. The supernatant was replaced with fresh PBS of equal volume. The amount of BSA released at the indicated periods analyzed using QuantiPro™ BCA Assay Kit.
- Cellular bioactivity study Cells were subcultured (i.e. trypsinized) and seeded in a 96 well plate at equal concentration. Rabbit vascular smooth muscle cells (RVSMC) and human dermal fibroblasts (HDF) were studied, respectively. Cells were grown in cell culture medium for 48 hours and serum-free medium (containing 0.10 % BSA) for another 24 hours. 20 μl of treatments (i.e. release medium) were added and incubated with the cells. Effect of the treatments on proliferation was analyzed for 48 hours for HDF and 72 hours for RVSMC. After the indicated time of study, the medium was replaced with 100 μl fresh serum-free medium (i.e. no BSA or FBS was added). 20 μl of CellTiter aqueous one solution (Promega,WI ) was then added for cell proliferation analysis. The plate was incubated covered and incubated at 37°C for 4 hours. Absorbance was read at 490nm with using a Wallac microplate reader. Percent proliferation of cells was based on the absorbance reading of the control wells, which contained serum-free medium only as a treatment.
- Characterization Morphology of the impregnated grafts and uncoated grafts were studied before and after incubation in PBS using a JEOL 6400 scanning electron microscope
RESULTS AND DISCUSSION

Fig. 2 Morphology of coated vascular grafts. A) Plain graft, B) 0.6% modified vascular graft, C) 1.2% modified vascular grafts, D) BSA modified vascular graft (x500 , 15kV)
The amount of added coating on the modified vascular graft ranged from 3.51-3.65 mg. There was no significant difference between the modified graft prepared with 0.6% sucrose compared to 1.2%. In addition, the morphology of the grafts were similar. The only difference observed was an increase in porosity of the graft modified with 1.2% sucrose. The morphology of the modified grafts can be found in figure 2 below
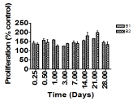
Release kinetics:

The trend of percent weight loss of the coated grafts coated with higher concentrated sucrose, B2, was similar to bovine serum coated (BSA) graft (B3 ) without sucrose and the excess protein additives. The increase in the amount released might be due to the fact that the higher concentration sucrose increases water uptake, which would lead to increase in the weight loss. The triphasic drug release profile observed with the weight loss, was also observed with the concentration of BSA released for B2 coated graft.). However, with the higher weight loss observed with B2 coated graft, the slower the release of BSA. An initial burst was observed for the graft coated with 0.6% sucrose (B1) with most of the protein (85%) released by the 7th day (Figure 3).
Cellular bioactivity